PRODUCT NAME
Common Name: SARS-CoV-2 & Influenza A/B & RSV Antigen Combo Test Kit (Colloidal Gold
Chromatographic Immunoassay)
MF-71
WHAT DOES THE KIT TEST?
The
fluorecare® SARS-CoV-2 & Influenza A/B & RSV Antigen Combined Test Kit
is applicable to the simultaneous
qualitative detection and differentiation of novel Coronavirus (SARS-CoV-2 Antigen),
Influenza A virus, Influenza B virus Antigen
and/or RSV Antigen in population
Nasal swabs samples in vitro.
It can be used as an aid to diagnose coronavirus infection disease (COVID-19), caused by SARS-CoV-2, in symptomatic patients
within 7 days of onset. It can also be used to
aid in the diagnosis of diseases caused by Influenza A/B or RSV.
For in vitro diagnostic use only. For self-testing use.
Specifications
1 Test/boxïž2 Tests/box ,5 Tests/box
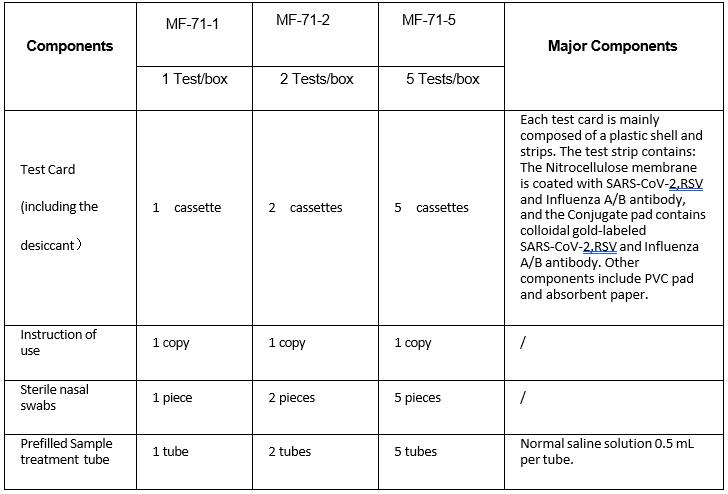
MAKE SURE YOUR TEST KIT CONTAINS
1.
Test Card
2.
Sample treatment
solution
3.
Sterile nasal
swabs
4.
Sample treatment
tube
User age
requirement
This kit is suitable for people over 2 years old.
People under
the age of 2-14 cannot operate by themselves. This kit should
be used by adults or parents (18-60 years old) for
sample collection and testing.
People aged 14-17 can use this kit to collect
samples and test samples under the supervision of adults or parents (18-60 years old). Supervisors should
ensure that users have a detailed understanding of the requirements of the instructions and watch whether
the user's operation is correct.
For people over 75 years old, it is recommended that family members or guardians (18-60 years old) use this kit to collect
samples and test samples.
BACKGROUD
The novel coronaviruses belong to the Îē genus.
COVID-19 is an acute respiratory infectious
disease. People are generally susceptible. Currently, the patients infected by the
novel coronavirus are the main source of infection. Asymptomatic infected
people can also be an infectious source.
Based on the current epidemiological investigation, the incubation period is 1 to 14 days, mostly
3 to 7 days. The main manifestations include
fever, fatigue and dry cough. Nasal congestion, runny nose, sore throat,
myalgia and diarrhea are found in a
few cases.
Influenza (flu) is a contagious respiratory
illness caused by influenza viruses. Influenza
viruses can cause mild to severe illness. Serious outcomes of the flu
can result in hospitalization or death. Some people, such as older people, young children, and people with certain underlying health conditions, are at higher risk for serious flu complications. There are two main types of influenza
viruses: types A and B. Both type A and B influenza
viruses regularly spread in people, and are responsible for seasonal flu each year. Influenza viruses can be spread to
others before and after a person shows signs
and symptoms of being sick.
Respiratory syncytial virus (RSV) belongs to the
genus Pneumovirus of the family Paramyxoviridae.
It can be infected by coughing and air droplets, mainly causing lower respiratory tract infections such as
bronchiolitis and pneumonia in infants under 6
months, and upper respiratory tract infections such as rhinitis and cold
in older children and adults, and
bronchitis or pneumonia in the elderly.
PRINCIPLE
The SARS-CoV-2 & Influenza A/B & RSV
Antigen test is a qualitatively test to detect
SARS-CoV-2 Antigen /
Influenza A/B Antigen/RSV Antigen in Nasal swabs samples by the colloidal gold method. After sample
added, the SARS-CoV-2 Antigen (or Influenza A/B
& RSV) in the sample to be tested is combined with the SARS-CoV-2 (or
Influenza A/B & RSV) antibody
labelled with colloidal gold on the Conjugate pad to form the SARS-CoV-2 Antigen (or Influenza A/B
& RSV) antibody-colloidal gold complex. Due to chromatography, the SARS-CoV-2
Antigen (or Influenza A/B & RSV)-antibody-colloidal gold complex diffuses along the
nitrocelluloseâs membrane. Within the detection line area, the SARS-CoV-2 Antigen
(or Influenza A/B &
RSV)-antibody complex binds to the antibody
enclosed within the detection line area, showing a purple-red band. Colloidal gold labelled SARS-CoV-2 (or Influenza A/B
& RSV) antibody diffuses to the quality control
line (C) region and is captured by Goat anti-mouse
IgG to form red bands. When the
reaction is over, the results can be interpreted by visual observation.
LIMITATION OF METHODOLOGY
1.
This kit is a qualitative test and is only used for
in vitro auxiliary
diagnosis.
2. Negative test results may
occur if the level of antigen in a sample is below the detection limit of the test, or from improper sample collection,
and the negative results are not
intended to exclude other non COVID-19 virus, Influenza virus or RSV virus infections.
3. Unreasonable sampling,
transportation, handling, and low virus content in samples may lead to false negatives.
4. This reagent is a qualitative assay. As
it is with any diagnostic procedure, a confirmed
virus infection diagnosis should only be made by a physician after
evaluating all clinical and
laboratory findings.
5. Reading the test results
earlier than 15 minutes or later than 20 minutes may give incorrect
results.
6. A negative test result for COVID-19, Influenza A/B or RSV Antigen does not rule out COVID-19,
Influenza A/B or RSV infection and does not exempt you from the applicable rules for spread control (e.g. contact
restrictions and protective measures).
WARNING AND PRECAUTION
1. Read the Instruction for use completely before using the product. Follow
the instructions carefully. Failure to do so may result in
an inaccurate result.
2. The kit is only used for in vitro diagnosis; it cannot be used repeatedly. Do not swallow.
3. Avoid getting the buffer solution
into the eyes or skins.
4. Keep out of reach children.
5. The test kit is for single use only, do not reuse any components of the test kit.
6. Do not use this test beyond
the expiration date printed on the outer package. Always check expiry date prior to testing.
7. Do not touch the reaction
area of the test cassette.
8. Do not use the
kit if the pouch is punctured or not well sealed.
9.
DIPOSAL: All specimens and the used-kit has the infectious
risk. The process of disposing the
diagnostic kit must follow the local, state and federal infectious disposal laws/regulations.
11.
During the time of interpretation, no matter the shade of the color band, it can be found to be positive as long as two lines appear
on the quality control area and the detection
area, respectively.
12.
Please ensure that an appropriate amount of sample is used
for testing, too much or too little
of sample amount will cause the result deviation.
13. The final result should be read in 15 minutes. Please do not read the result after 20 minutes.
14. Various components of different batch
of reagents cannot be used interchangeably in order to avoid wrong results
STORAGE CONDITION AND EXPIRY DATE
1.Test kit store at 2-30â in dry place and protect from light. Test kit is valid for 18 months. 2.The Test Card must remain in the sealed
pouch until use. Once the test card pouch is opened, the test
should be performed within 1 hour.



