ŃĆÉ õĖćÕŁÜ┬Āµ¢░Õ×ŗÕåĀńŖČńŚģµ»Æ’╝ł2019-nCoV’╝ēµŖŚÕĤµŻĆµĄŗĶ»ĢÕēéńøÆ’╝łĶāČõĮōķćæµ│Ģ’╝ē ŃĆæ
Wondfo 2019-nCoV Antigen Test (Lateral Flow Method) is a rapid test that is used for laymen of detecting novel coronaviruses (2019-nCoV) N protein antigen extracted from the nasal swab specimen. It is intended as an aid in the diagnosis of coronavirus infection disease (COVID-19) for asymptomatic patients and/or symptomatic patients within 7 days after onset of symptoms, which is caused by 2019-nCoV. For in vitro diagnostic use only. For self-testing use. According to usability study on laymen user, the test can be correctly performed for anyone age over 18. However, children under the age of 18 should be supported or under the supervision by an adult.
SPECIFICATIONS
|
Components |
W634P0024 |
W634P0028 |
W634P0025 |
W634P0027 |
|
Sealed Pouch(pcs) |
1 |
2 |
5 |
20 |
|
Extraction Buffer |
1 |
2 |
5 |
20 |
|
Disposable Sterile Swab(pcs) |
1 |
2 |
5 |
20 |
|
Waste Bag(pcs) |
1 |
2 |
5 |
20 |
|
Instruction for Use(pcs) |
1 |
1 |
1 |
1 |
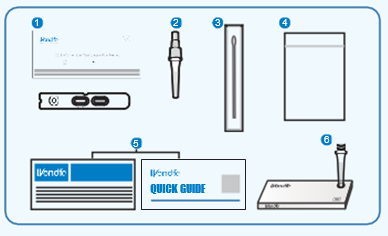
MAKE SURE YOUR TEST KIT CONTAINS
1. Sealed Pouch
2. Extraction Buffer (400╬╝L)
3. Disposable Sterile Swab
4. Waste Bag
5. Instruction for Use
6. TubeRack (in the outer box
LIMITATIONS OF PROCEDURE
1. This reagent is designed to detect 2019-nCoV antigen in human nasal
swab specimen.
2. Failure to follow the instructions for the test procedure and interpretation of
test results may adversely affect test performance and/or produce invalid
results.
3. Reading the test results earlier than 15 minutes or later than 20 minutes
may give incorrect results.
4. The sample collection process will affect the accuracy of the test, such as
improper sample collection, improper sample storage, etc.
5. This reagent is a qualitative assay. As it is with any diagnostic procedure,
a confirmed 2019-nCoV infection diagnosis should only be made by a
physician after evaluating all clinical and laboratory findings.
6. Negative test results may occur if the level of antigen in a sample is below
the detection limit of the test, or from improper sample collection, and the
negative results are not intended to exclude other non 2019-nCoV virus
infections.
7. A negative test result does not rule out a coronavirus infection and does not
exempt you from the applicable rules for spread control (e.g. contact
restrictions and protective measures). Symptomatic patient must seek
further testing (e.g. immediate PCR), even though negative result occurs.
8. Positive test results do not exclude co-infections with other pathogens or identify specific 2019-nCoV virus subtype (like SARS-CoV virus), and
cannot necessarily determine whether a person is infectious.
9. SARS-CoV virus variants including Delta (B.1.617.2) and Omicron
(B.1.1.529) have been detected out by Wondfo 2019-nCoV Antigen Test
(Lateral Flow Method).
STORAGE AND STABILITY
1. The test kit should be stored at 2~30┬░C (storage in refrigerator is
permitted). Do not store the kit in the freezer. Improper storage may result
in an inaccurate result.
2. The test cassette is sensitive to humidity and temperature. Once removed
from foil pouch, test cassette is stable for up to 1 hour.
3. The test kit is stable until the expiration date printed on outer package. Do
not use it beyond the expiration date.
4. The test cassette must remain in the sealed pouch until use.
-1.jpg)













 13632887203
13632887203